The modern era of neuromodulation began in the early 1960s, first with deep brain stimulation which was soon followed (in 1967) by spinal cord stimulation, both for otherwise intractable pain.
Neuromodulation
What is Neuromodulation ?
-
The gradual realization that pain was the result of complex dynamic processes in the nervous system and not simply the result of activity in a hard-wired system was greatly enhanced by the publication of the Gate Theory in 1965. As damage to the nervous system can itself cause chronic pain, there began a gradual move away from destructive surgical treatments such as cutting nerves and towards reversible, modulatory treatments.
-
The International Neuromodulation Society defines therapeutic neuromodulation as “the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body.” In appropriate patients, this growing class of therapies, in common use since the 1980s, can help restore function or relieve symptoms that have a neurological basis.
Why Neuromodulation ?
Well-tolerated and minimal/no side effects
Neuromodulation is often well-tolerated, and the side effects pretty minimal. Some neuromodulation devices can be somewhat painful on the skin when they are used, but the main downside could be ineffectiveness. Sometimes, you can see additional benefits when using neuromodulation with medications that you’re currently taking.
Avoiding the use of medications
If you’re not on medication, neuromodulation can help you gain preventive benefits without committing to a certain medication. This ability to avoid medication is why neuromodulation attracts so many patients.
Safe in pregnancy
Some of these neuromodulation treatments are safe alternatives during pregnancy. While most of these devices have not been studied during pregnancy to measure its safety benefits, avoiding other medications in favor of neuromodulation is, so far, considered to be the safer option.
Vagus Nerve Stimulation
The vagus nerve is the major parasympathetic branch of the autonomic nervous system, whose primary functions include regulation of breathing, heart rate, and digestion.
The vagus nerve is a mixed nerve composed of 20% “efferent” fibers and 80% “afferent” fibers.
An important function of the vagus nerve is transmitting and/or mediating sensory information from throughout the body to the brain. Nociceptive transmission is probably modulated through the activation of vagus nerve afferents that go to the nucleus tractus solitarius, the area postrema and spinal trigeminal nucleus in the brainstem and spread to higher structures including the locus coeruleus, the periaqueductal grey, the raphe magnus, the thalamus and hypothalamus.
Implanted vagus nerve stimulation (iVNS) is mainly used to treat refractory epilepsy and (less frequently) drug-resistant depression. The impact of iVNS on seizure-associated headaches was first reported over 15 years ago. The mechanism of action of iVNS in the treatment of headache is largely elusive and likely multifactorial. In animal models, iVNS is able to modulate the firing of trigeminal nucleus caudalis neurons in response to dura mater stimulation.
Now, new noninvasive vagus nerve stimulation (nVNS) of the cervical branch at the neck (GammaCore®, electroCore LLC, Basking Ridge, NJ, USA) or of the auricular branch at the concha of the outer ear (Nemos®, Cerbomed, Erlanger, Germany) has been developed. The cervical nVNS device produces a low-voltage electrical signal (5- kHz sine wave series that occurs for 1 ms and is repeated every 40 ms [25 Hz]) that delivers a maximum output current of 24 V and 60 mA. Two stainless steel contact surfaces coated with a conductive gel enable delivery of stimulation to the neck in the vicinity of the vagus nerve.
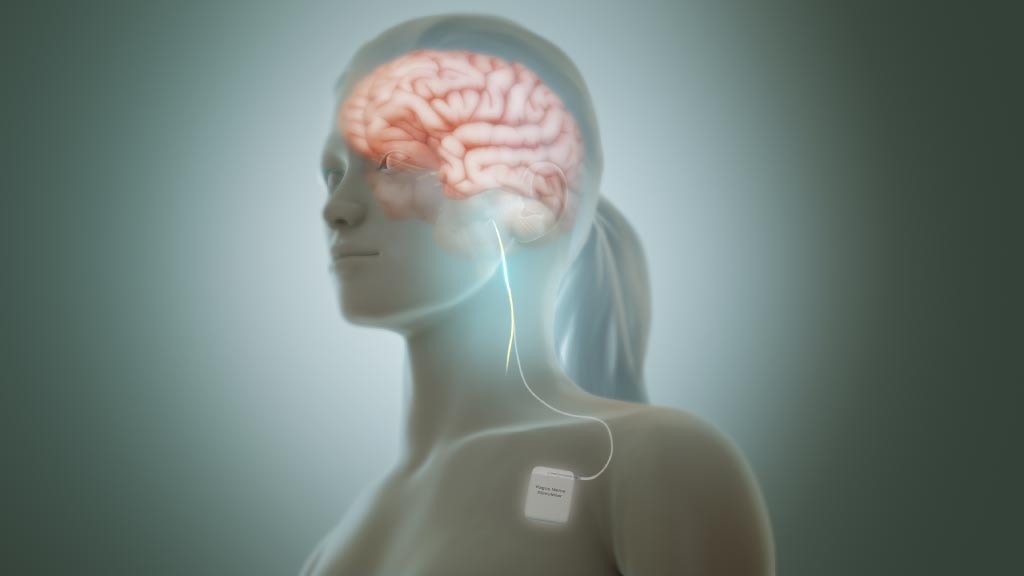
The transcutaneous auricular VNS device consists of a handheld, battery-driven electrical stimulator connected to an ear electrode placed in contact with the skin of the concha. Series of electrical pulses (pulse width: 250 μs, frequency: 1 Hz or 25 Hz, duty cycle: 30s on, 30s off, to avoid habituation) are applied to the skin of the concha. Future guidelines currently under development should help clinicians make informed decisions on appropriate treatment for their patients with these therapies.
There is quite a lot of research reporting that neuromodulation, such as with SCS (spinal cord stimulation) and ONS (occipital nerve stimulation), can be beneficial for refractory CM. Furthermore, the application and clinical effects of trigeminal nerve stimulation, transcranial magnetic stimulation, vagus nerve stimulation, and cerebellar stimulation, etc., are also under study and being explored (1).
Peripheral Nerve Field Stimulation (PNFS)
- Different from SCS (spinal cord stimulation) where electrodes are placed in the epidural space, and also unlike peripheral nerve stimulation (PNS) where electrodes are placed near a specific peripheral nerve, PNFS therapy does not target specific nerves that dominate the corresponding pain area.
- Instead, the electrodes are placed in the center of the pain region to stimulate the affected nerve in the area, including the skin sensory afferent nerves and segmental spinal nerve branches (12). PNFS is different from SCS and PNS in terms of therapeutic mechanisms, clinical application, characteristics, key points of operation, and parameter settings.
The mechanism of PNFS in the treatment of chronic migraine
At present, the mechanisms of chronic migraine (CM) are still unclear. It is currently accepted that it may be related to abnormal pain regulation, central sensitization, cortical hyperactivity, and neurogenic inflammatory response.
As for the chronicity of migraine, it is a gradual process. Recurrent headaches lead to the activation of the trigeminal vascular system and the weakening of the function of the brain stem descending inhibition system, resulting in increased cortical excitability, ultimately resulting in repeated episodes of pain. (13)
The mechanisms of PNFS treating pain are also unclear. Similar to SCS and PNS, it is believed to be based on the “Gate Theory” put forth by Melzack and Wall (14) which suggest that by stimulating the myelinated A-fibers, the glial inhibitory interneurons (SG cells) are stimulated, thus closing the gate and inhibiting the activity of the dorsal horn projecting neurons (T cells), and subsequently inhibiting the transmission of nociceptive information to the high center to alleviate pain.
In addition to these effects on the segmental regulation of the spinal cord, some researchers believe that PNFS may also affect the descending pain modulation system of the upper central nervous system by stimulating the subcutaneous electrode, thus exerting an analgesic effect. In addition, some studies have shown that local electrical stimulation can reduce the inflammation of the cutaneous nerve fibers, depolarizing the cell membrane, and reducing the sensitivity of catecholamine in the blood (15-17).
Moreover, the possible mechanisms for PNFS also include influencing the excitability of the receptors and tissues in the pain area; affecting the local blood circulation; regulating the level of neurotransmitters; affecting the conduction of the spinal and thalamus pathway and affecting the sympathetic efferent function (18). Although there is no unified conclusion on the mechanisms of PNFS, most researchers believe that PNFS may impact the endogenous enkephalin level, thus changing the threshold of nociceptive sensation in the target area. Therefore, further research into its basic mechanism is needed (19-20).
References
- Roceanu A, Antochi F, Bajenaru O. Chronic migraine – new treatment options. Maedica. 2014;9(4):401–404.
- Abejón D, Krames ES. Peripheral nerve stimulation or is it peripheral subcutaneous field stimulation; what’s in a moniker? Neuromodulation. 2009;12(1):1–4. doi:10.1111/j.1525- 1403.2009.00192.x
- Slavin KV, Yin D, Yin H, Gomez C. Subcutaneous peripheral nerve field stimulation for intractable pain. In: Neuromodulation. 2 nd ed. Academic Press; 2018;523–528.
- Headache Classification Committee of the International Headache Society (IHS),The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. doi:10.1177/0333102413485658
- Young WB. Occipital nerve stimulation for chronic migraine. Curr Pain Headache Rep. 2014;18(2):396. doi:10.1007/s11916-013-0396-x
- Perini F, De Boni A. Peripheral neuromodulation in chronic migraine. Neurol Sci. 2012;33(Suppl 1):S29–S31. doi:10.1007/s10072-012-1039-4
- Mekhail NA, Estemalik E, Azer G, Davis K, Tepper SJ. Safety and efficacy of occipital nerves stimulation for the treatment of chronic migraines: randomized, double-blind, controlled single-center experience. Pain Pract. 2017;17:669–677. doi:10.1111/papr.12504
- Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86. doi:10.1186/1129-2377-14-86
- De Agostino R, Federspiel B, Cesnulis E, Sandor PS. High-cervical spinal cord stimulation for medically intractable chronic migraine. 2015;18(4):289–296; discussion 296. doi:10.1111/ner.12236
- Arcioni R, Palmisani S, Mercieri M, et al. Cervical 10 kHz spinal cord stimulation in the management of chronic, medically refractory migraine: a prospective, open-label, exploratory study. Eur J Pain. 2016;20(1):70–78. doi:10.1002/ejp.692
- Chichorro JG, Porreca F, Sessle B. Mechanisms of craniofacial pain. Cephalalgia. 2017;37(7):613–626. doi:10.1177/0333102417704187
- Barolat G. Subcutaneous peripheral nerve field stimulation for intractable pain. In: Krames E, Peckham PH, Rezai A, editors. Neuromodulation. San Diego (CA): Elsevier; 2009:1017–1020.
- Schwedt TJ, Larsonprior L, Coalson RS, et al. Allodynia and descending pain modulation in migraine: a resting state functional connectivity analysis. Pain Med. 2014;15(1):154–165. doi:10.1111/pme.12267
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971. doi:10.1126/science.150.3699.971
- Mørch CD, Nguyen GP, Wacnik PW, Andersen OK. Mathematical model of nerve fiber activation during low back peripheral nerve field stimulation: analysis of electrode implant depth. Neuromodulation. 2014;17:218–225. doi:10.1111/ner.12163
- Reverberi C, Bonezzi C, Demartini L. Peripheral subcutaneous neurostimulation in the management of neuropathic pain: five case reports. Neuromodul. 2009;12:146–155. doi:10.1111/j.1525- 1403.2009.00201.x
- Krutsch JP, McCeney MH, Barolat G, Al Tamimi M, Smolenski A. A case report of subcutaneous peripheral nerve stimulation for the treatment of axial back pain associated with postlaminectomy syndrome. 2008;11:112–115. doi:10.1111/j.1525-1403.2008.00151.x
- Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60(11):1524–1534. doi:10.1001/archneur.60.11.1524
- Ellrich J, Lamp S. Peripheral nerve stimulation inhibits nociceptive processing: an electrophysiological study in healthy volunteers. 2005;8:225–232. doi:10.1111/j.1525-1403.2005.00029.x [PubMed]
- O’Neill CW, Liu JJ, Leibenberg E, et al. Percutaneous plasma decompression alters cytokine expression in injured porcine intervertebral discs. Spine J. 2004;4:88–98. doi:10.1016/S1529-9430(03)00423-6
